-
PDF
- Split View
-
Views
-
Cite
Cite
Eiichi Fukushima, Yuuto Arata, Tsuyoshi Endo, Uwe Sonnewald, Fumihiko Sato, Improved Salt Tolerance of Transgenic Tobacco Expressing Apoplastic Yeast-Derived Invertase, Plant and Cell Physiology, Volume 42, Issue 2, 15 February 2001, Pages 245–249, https://doi.org/10.1093/pcp/pce027
Close - Share Icon Share
Abstract
We investigated the salt tolerance of transgenic tobacco, in which yeast invertase is expressed in the apoplastic (Apo-Inv) spaces. Whereas photosynthetic activities in wild-type tobacco in light were inhibited under salt stress, transgenic Apo-Inv tobacco maintained constant photosynthetic activities. The physical appearance of plants under salt stress also indicates that yeast invertase expression in the apoplastic space is beneficial for inducing salt tolerance. Apo-Inv tobacco had a much higher osmotic pressure increase in the cell sap than did wild-type tobacco under this type of stress. The physiological importance of sucrose metabolism under salt stress is discussed.
(Received August 21, 2000; Accepted December 6, 2000).
To clarify stress tolerance in plants and to improve plant productivity, various investigations have been made of gene expression in plants under water stresses (e.g. drought, salt, freezing), the most severe limiting factors of plant growth and yields. Under stress conditions, plants accumulate a set of proteins, such as LEAs, and low molecular weight compounds, so-called ‘compatible solutes’ (see review, Bohnert et al. 1995, Ingram and Bartels 1996). Compatible solutes are compounds that accumulate in stress-tolerant plants under water stresses. They are water soluble and do not disturb plant cell metabolism. Several compatible solutes are known: sugars, polyols, amino acids, and amino acid derivatives. These are involved in osmoregulation, removal of free radicals (Smirnoff and Cumbes 1989, Shen et al. 1997), and stabilization of the hydrated structure of proteins to maintain membrane integrity and protein stability (Inchroensakdi et al. 1986, Papageorgiou et al. 1991, Murata et al. 1992). Introduction of genes for biosynthesis of compatible solutes has been used successfully to improve the stress tolerance of plants (Tarczynski et al. 1993, Hayashi et al. 1997, Sheveleva et al. 1998).
Very few reports, however, have dealt with the in vivo function of sucrose metabolism and its effects on photosynthesis under water stress, whereas stress causes an increase in the sucrose concentration in water-stressed plants, and sucrose synthase and sucrose phosphatase genes are up-regulated (Ingram and Bartels 1996). Furthermore, recent QTL investigation suggested that some invertase activities are closely related to water stress responses (Pelleschi et al. 1999). We therefore investigated the salt tolerance of transgenic tobacco plants that express yeast invertase in the apoplast (Apo-Inv) or vacuole (Vac-Inv) and that accumulate more sucrose and hexoses in the leaves in light than does wild-type tobacco (von Schaewen et al. 1990, Sonnewald et al. 1991, Heineke et al. 1994). In these transformants, sugar transportation to the sink organ has been inhibited by ectopically expressed yeast invertase in the apoplastic space or vacuole, resulting in accumulation of sucrose in the cytoplasm of the source organ. The sucrose remained in cytoplasm of mesophyll cells would be in part converted to glucose and fructose. The sucrose contents in Apo-Inv and Vac-Inv tobacco are reported to be respectively 15 and 5 times that in wild-type tobacco, whereas glucose contents are 50 and 90 times greater, and fructose contents 61 and 25 times greater (Sonnewald et al. 1991, Buessis et al. 1997). It is expected that high sugar contents in these transformants may protect photosynthetic apparatus under salt stress.
Plant culture conditions and salt stress treatment
Transgenic tobacco (Nicotiana tabacum L. cv. Samsun NN) plants, which express yeast invertase in apoplastic and vacuolar spaces, as well as wild-type tobacco seedlings were maintained aseptically on agar media containing a half concentration of Linsmaier-Skoog (LS) inorganic medium (Linsmaier and Skoog 1965) and cultured for 2 weeks in hydroponic medium with a quarter concentration of LS at 25°C under continuous light (100 µmol quanta m–2 s–1) with aeration. Salt tolerance was evaluated under moderate light intensity (200 µmol quanta m–2 s–1) in hydroponic medium with a quarter concentration of LS with or without 300 mM NaCl. The plants were pre-adapted to the continuos light illumination at 200 µmol quanta m–2 s–1 for at least 1 week before salt stress treatment.Photosynthetic activities were measured with a PAM2000 Chl fluorometer (Heinz Walz, Effeltrich, Germany). The estimated activities were the quantum yield of photosystem II (ΦII = ΔF / Fm’ = (Fm’ – F) / Fm’) under white actinic light of 200 µmol quanta m–2 s–1 (Genty et al. 1989); potential activity of photosystem II (Fv/Fm, Fv = Fm – Fo) after 15 min of dark adaptation.
Photosynthetic activities in Apo-Inv tobacco plants under salt stress
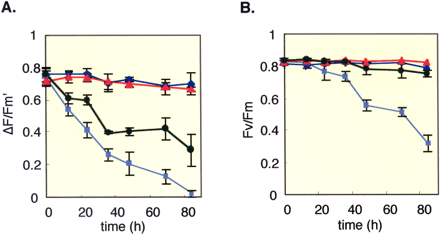
Photosynthetic activity in transgenic tobacco plants that expressed yeast invertase was evaluated under salt stress and compared with that in wild-type plants (Fig. 1A). In the latter, the quantum yield of PSII (ΦII), indicated by the Chl fluorescence parameter ΔF/Fm’, was markedly decreased by salt stress, whereas the ΦII of Apo-Inv tobacco remained as high as that of the non-stressed control. In contrast, under salt stress the ΦII of Vac-Inv tobacco decreased more sharply than that of wild-type tobacco (data not shown). The reason for the different photosynthetic activity responses of Apo-Inv and Vac-Inv tobacco is not clear, but this result suggests that not only sucrose accumulation, but the mechanism of accumulation (localization of invertase) is important for the growth performance of plants as indicated by the more severe defect in Vac-Inv tobacco than Apo-Inv tobacco even under normal growth conditions. In the experiments described below, we used Apo-Inv tobacco to further characterize the function of sucrose metabolism in vivo.
The degree of photoinhibition under salt stress
Apo-Inv tobacco showed tolerance to salt stress and had a higher quantum yield of PSII than wild-type tobacco under the same stress (Fig. 1A). To confirm the tolerance under salt stress, we measured the degree of photoinhibition in wild-type and Apo-Inv plants under salt stress. The degree of photoinhibition (degradation of PSII core protein D1) can be estimated from the Chl fluorescence parameter Fv/Fm (Schreiber and Bilger 1993). Salt stress clearly produced different degrees of photoinhibition in the wild-type and Apo-Inv plants (Fig. 1B). Wild-type tobacco showed marked photoinhibition due to salt stress, whereas no apparent photoinhibition occurred in Apo-Inv tobacco. This difference is attributable in part to the difference in the reduction level of QA (data not shown), because acceptor-side photoinhibition is induced by the reduction of QA (Barber 1994). Alternatively, the reaction center protein may be protected from photo-degradation in chloroplasts of Apo-Inv tobacco.
Figure 2 shows the physical appearance of Apo-Inv and wild-type tobacco plants stressed in the presence of 300 mM NaCl for 122 h. The Apo-Inv plants appears to be much healthier than the wild-type one because there is less photodamage. The more turgid appearance of the Apo-Inv plant may be due to higher osmotic pressure in the cells (see below; Fig. 3).
Osmotic pressure
The osmotic pressure in the cell varies with the plant species, developmental stage, and environment. The generation of osmotic pressure is essential for maintaining turgor pressure for the absorption of water and for keeping the cell shape under salt/water stress. Because Apo-Inv tobacco plants had a better physical appearance under salt stress, the osmotic pressure in leaf cells with and without salt treatment was determined. Fresh leaves were frozen in liquid nitrogen and then thawed in warm water. After centrifugation of the mixture at 1,000×g for 5 min, the leaf pellets obtained were squeezed with a teaspoon, and the cell sap was collected. The osmotic pressure of the sap was measured with an osmometer (Shimadzu, model OSM-1, Kyoto, Japan).
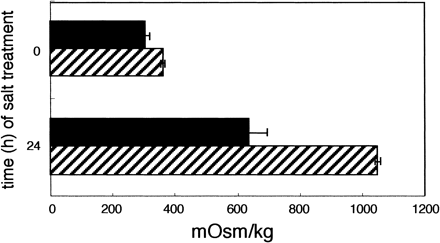
Osmotic pressures in both the wild-type and Apo-Inv tobacco were similar under the non-stress condition, but salt stress at 0.3 M NaCl for 24 h clearly increased the osmotic pressure in Apo-Inv tobacco, from ca. 400 to ca. 1,050 mOsm kg–1, whereas in wild-type tobacco the increase was only from ca. 300 to ca. 600 mOsm kg–1 (Fig. 3). This suggests that Apo-Inv tobacco maintained much higher turgor pressure for the absorption of water from medium (0.3 M NaCl; ca. 600 mOsm kg–1).
Concentration of sucrose and hexoses in chloroplasts
Figures 1 and 2 patently show that the photosynthetic apparatuses in Apo-Inv tobacco were well protected under salt stress. What then is the agent that protects photosynthetic activity? It is generally believed that sucrose does not appear inside the plastids (Avigad and Dey 1997), but there are reports of the presence of sucrose inside chloroplasts (Heineke et al. 1994, Lohaus et al. 1999) and that an increase in the sucrose concentration in the leaves of transgenic tobacco with yeast-derived invertase increases the sucrose concentration in the chloroplasts (Heineke et al. 1994). However, there have been no reports on the sucrose concentration in chloroplasts under salt stress. We therefore measured the sucrose concentration in chloroplasts of both the wild-type and Apo-Inv plants under salt stress.
To prepare intact chloroplasts, leaves were homogenized at 4°C in buffer A (a concentration of sorbitol isotonic to the osmotic pressure in the leaves with 50 mM of MES, pH 6.1, and a teaspoon of ascorbate) in a Waring blender for 3 s at maximum speed. The homogenate was filtered through four layers of gauze, and chloroplasts obtained were quickly centrifuged at 2,000×g for 30 s and then suspended in buffer B (isotonic concentration of sorbitol and 50 mM of HEPES, pH 7.6). Intact chloroplasts were separated on 40% and 75% Percoll buffer layers by centrifugation at 2,000×g for 5 min and recovered between the 40% and 75% Percoll surface. To evaluate chloroplast intactness, oxygen evolution (10–20 (mg Chl) ml–1) in buffer B supplemented with 1 µM nigericin and 0.5 mM potassium ferricyanide was measured with a Clark type oxygen electrode (Hansatech, U.K.) under 2,500 µmol quanta m–2 s–1 light. Oxygen evolution in the broken chloroplasts was measured as described above, and intactness calculated from the difference in activities. Chloroplasts were suspended in 80% acetone at 4°C to measure Chl concentration. Arnon’s equation (Arnon 1949) was used to calculate the Chl concentration from the absorbances of the extracts.
To measure the sugar content, intact chloroplasts were frozen at –20°C then thawed and thoroughly broken by sonication (max power, 5 s). Proteins were removed from the chloroplast suspension by the Carrez reagent. The chloroplast suspension (600 ml) was mixed vigorously with 50 ml of Carrez I (ferrocyanide, final concentration, 4.25 mM) and 50 ml of Carrez II (zinc sulfate, final concentration, 12.5 mM). After centrifugation of the mixture at 20,000×g for 15 min, the supernatant was filtered through a MILLIPORE GV membrane and desalted for 1 h with Amberlite IRN-150L resin (Pharmacia, Plusone). The concentrations of sucrose and hexoses in the supernatant were measured with an enzymatic assay kit (Roche Diagnostics K.K., TC Sucrose/d-Glucose/d-Fructose) according to the manufacturer’s protocol.
Chloroplast volume in wild-type tobacco (osmotic pressure ca. 300 mOsm kg–1) was assumed to be 95 µl (mg Chl)–1 on the basis of reported values (Winter et al. 1993, Winter et al. 1994) to calculate sugar concentration. The chloroplast volume in both tobacco types was estimated from the inverse logarithmic relationship of the volume of intact spinach chloroplasts and the sorbitol concentration in the medium considered to be the osmotic pressure in the cytosol (Heldt and Sauer 1971). The sugar concentration in chloroplasts was calculated based on the intactness of the chloroplasts, sugar content (µmol sugar (mg Chl)–1), and chloroplast volume (µl (mg Chl)–1).
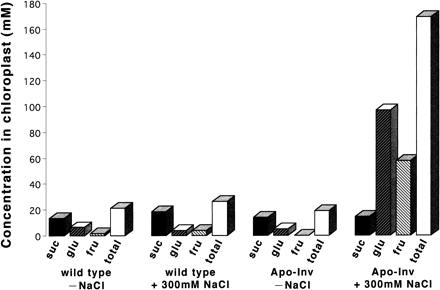
Figure 4 shows that sucrose was present in both the wild-type and Apo-Inv tobacco chloroplasts. Estimated sucrose contents in the chloroplasts of both types without salt stress were similar to those reported previously (Heineke et al. 1994, Lohaus et al. 1999). After 24 h of salt stress, the estimated sucrose content in wild-type tobacco had increased slightly whereas in Apo-Inv tobacco with salt stress it was similar to the value without salt stress. In contrast, the hexose content of Apo-Inv tobacco showed a marked increase after salt stress, whereas without salt stress they were much lower than the sucrose content. The hexose content of wild-type tobacco were not altered by salt stress. The chloroplasts of salt-stressed Apo-Inv tobacco therefore had a much higher total of sucrose, glucose, and fructose than did those of the wild type with or without salt treatment and those of Apo-Inv without salt treatment. The estimated contribution of the sugars (180 mOsm kg–1) to osmotic pressure was relatively low in chloroplasts of salt-stressed Apo-Inv tobacco because the osmotic pressure of the leaf was about 1,050 mOsm kg–1. This marked increase in the sugar concentration in chloroplasts of Apo-Inv tobacco during salt stress is, however, important for the adjustment of osmotic balance in the chloroplasts and providing compatible solutes for the protection of photosynthesis.
Concluding remarks
In Apo-Inv plants, a large accumulation of sugars, mostly in the form of glucose and fructose, in chloroplasts under salt stress was observed. Inhibition of sugar transportation to the sink organ in Apo-Inv plants resulted in accumulation of sucrose in source organ, and a part of remained sucrose seemed be converted to glucose and fructose. It is reasonable to conclude that these changes in sucrose metabolism in Apo-Inv transformants protect photosynthetic apparatus under salt stress. Molecular mechanisms of the protection, however, remain to be clarified.
Acknowledgments
This work was supported in part by a Grant-in-Aid of Scientific Research on Priority Areas (No. 04273103) from the Ministry of Education, Science, Culture and Sports, Japan (F.S); Grant JSPS-RFTF9616001 (F.S.); and a NEDO International Joint Research Program grant (F. S.).
Present address: Division of Integrated Life Sciences, Graduate School of Biostudies, Kyoto University, Kyoto, 606-8502 Japan.
Corresponding author: E-mail, fumihiko@kais.kyoto-u.ac.jp; Fax, +81-75-753-6398.
Fig. 1 Effects of salt stress on various Chl fluorescence parameters in Apo-Inv (circle and triangle) and wild-type (square and diamond) tobacco plants. Plants were treated with (square, circle) or without (diamond, triangle) 300 mM NaCl in light (200 µmol quanta m–2 s–1). Vertical bars are standard errors for three replicants. (A) quantum yield of PSII (ΦII, ΔF/Fm’), (B) photoinhibition of PSII (Fv/Fm).
Fig. 2 Physical appearance of Apo-Inv (left) and wild-type (right) tobacco plants under salt stress. After their lower leaves were removed, the plants were treated with 300 mM NaCl in light (200 µmol quanta m–2 s–1) for 122 h.
Fig. 3 Effects of salt stress on osmotic pressure in the leaf. Plants were treated with 300 mM NaCl in light (200 µmol quanta m–2 s–1) for 24 h. Closed bars are the wild-type and hatched ones Apo-Inv tobacco leaves. Horizontal bars standard errors for three replicants.
Fig. 4 Estimated sugar concentrations in chloroplasts before and after 24 h of salt stress (300 mM NaCl, 200 µmol quanta m–2 s–1) treatment. suc, sucrose; fru, fructose; glu, glucose; total = suc + fru + glu.
Abbreviations
- Apo-Inv
apoplastic invertase
- Apo-Inv tobacco
transgenic tobacco expressing yeast invertase in apoplastic space
- Fm
maximum yield of Chl fluorescence
- Fo
minimum yield of Chl fluorescence
- Fv
variable fluorescence Fm-Fo.
References
Arnon, D.I. (
Avigad, G. and Dey, P.M. (
Barber, J. (
Bohnert, H.J., Nelson, D.E. and Jensen, R.G. (
Buessis, D., Heineke, D., Sonnewald, U., Willmitzer, L., Raschke, K. and Heldt, H-W. (
Genty, B., Briantais, Y.M. and Baker, N. (
Hayashi, H., Alia, Mustardy, L., Deshnium, P., Ida, M. and Murata, N. (
Heineke, D., Wildenberger, K., Sonnewald, U., Willmitzer, L. and Heldt, H.W. (
Heldt, H.W. and Sauer, F. (
Inchroensakdi, A., Takabe, T. and T. Akazawa, T. (
Ingram, J. and Bartels, D. (
Linsmaier, E.M. and Skoog, F. (
Lohaus, G., Heineke, D., Kruse, A., Leidreiter, K., Riens B., Robinson, D.G., Winter, H., Winzer, T. and Heldt, W. (
Murata, N., Mohanty, P.S., Hayashi, H. and Papageorgiou, G.C. (
Papageorgiou, G.C., Fujimura, Y. and Murata, N. (
Pelleschi, S., Guy, S., Kim, J.Y., Pointe, C., Mahe, A., Barthes, L., Leonardi, A. and Prioul, J.L. (
Schreiber, U. and Bilger, W. (
Shen, B., Jensen, R.G. and Bohnert, H.J. (
Sheveleva, E.V., Marquez, S., Chmara, W., Zegeer, A., Jensen, R.G. and Bohnert, H.J. (
Smirnoff, N. and Cumbes, Q.J. (
Sonnewald, U., Brauer, M., von Schaewen, A., Stitt, M. and Willmitzer, L. (
Tarczynski, M.C., Jensen, R.G. and Bohnert, H.J. (
von Schaewen, A.V., Stitt, M., Schmidt, R., Sonnewald, U. and Willmitzer, L. (
Winter, H., Robinson, D.G. and Heldt, H.W. (