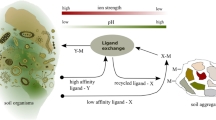
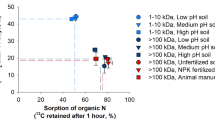
Abstract
Although iron is abundant in soils (1–6%), it is often unavailable to plants because its solubility is dependent on pH and controlled by the low solubility of ferric oxides. Iron availability to plant roots may thus depend on organic chelators which Lindsay reports would maintain an adequate iron supply by diffusion and mass flow at concentrations as low as 10−8 M1,2. Hydroxamate siderophores (HS), microbially produced, ferric-specific, iron transport molecules with stability constants3 as high as 1032, may represent the chelators which maintain these soil iron concentrations. Such peptide derivatives were shown to control iron availability in aquatic ecosystems4, but little is known about their soil role beyond their function as microbial growth factors5,6. We report here the occurrence of HS in aqueous extracts of a variety of soils in concentrations (10−7 M–10−8 M after correction to 10% soil moisture) sufficient to affect plant nutrition1,2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lindsay, W. L. in The Plant Root and Its Environment (ed. Carson, E. E.) 507–524 (University Press of Virginia, Charlottesville, 1974).
Lindsay, W. L. Chemical Equilibrium in Soils (Wiley, New York, 1979).
Anderegg, G., L'Eplattenier, F. & Schwarzenback, G. Helv. chim. Acta 46, 1400–1408 (1963).
Murphy, T. P., Lean, D. R. S. & Nalewajko, C. Science 192, 900–902 (1976).
Lochhead, A. G. & Burton, M. O. Soil Sci. 82, 237–245 (1956).
Waid, J. S. Soil Biochemistry Vol. 4 (eds Paul, E. A. & McLaren, A. D.) 65–101 (Dekker, New York, 1975).
Estep, M., Armstrong, J. E. & Van Baalen, C. Appl. Microbiol. 30, 186–188 (1975).
Lankford, C. E. Critical Rev. Microbiol. 2, 273–331 (1973).
Burnham, B. F. & Neilands, J. B. J. biol. Chem. 236, 554–559.
Csaky, T. Z. Acta chem. scand. 2, 450–454 (1948).
Bertin-Batsch, C. Ann. chim. Fr. 7, 481–531 (1952).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Powell, P., Cline, G., Reid, C. et al. Occurrence of hydroxamate siderophore iron chelators in soils. Nature 287, 833–834 (1980). https://doi.org/10.1038/287833a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/287833a0
This article is cited by
-
A pilot study on the effect of desferrioxamine B on uranium VI precipitation and dissolution in pH 11.5, 0.1 M NaCl solutions
Journal of Radioanalytical and Nuclear Chemistry (2022)
-
Chromium removal from aqueous solutions using new silica gel conjugates of desferrioxamine or diethylenetriaminepentaacetic acid
Environmental Science and Pollution Research (2020)
-
Increased Solubility and Bioavailability of Hydroxy-Cr(III) Precipitates in the Presence of Hydroxamate Siderophores
Bulletin of Environmental Contamination and Toxicology (2018)
-
Glyphosate, a chelating agent—relevant for ecological risk assessment?
Environmental Science and Pollution Research (2018)
-
A universal assay for the detection of siderophore activity in natural waters
BioMetals (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.