Abstract
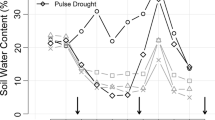
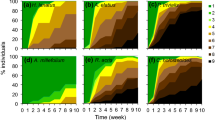
Quantifying plant drought resistance is important for understanding plant species' association to microhabitats with different soil moisture availability and their distribution along rainfall gradients, as well as for understanding the role of underlying morphological and physiological mechanisms. The effect of dry season drought on survival and leaf-area change of first year seedlings of 28 species of co-occurring woody tropical plants was experimentally quantified in the understory of a tropical moist forest. The seedlings were subjected to a drought or an irrigation treatment in the forest for 22 weeks during the dry season. Drought decreased survival and growth (assessed as leaf-area change) in almost all of the species. Both survival and leaf-area change in the dry treatment ranged fairly evenly from 0% to about 100% of that in the irrigated treatment. In 43% of the species the difference between treatments in survival was not significant even after 22 weeks. In contrast, only three species showed no significant effect of drought on leaf-area change. The effects of drought on species' survival and growth were not correlated with each other, reflecting different strategies in response to drought. Seedling size at the onset of the dry season had no significant effect on species' drought response. Our study is the first to comparatively assess seedling drought resistance in the habitat for a large number of tropical species, and underlines the importance of drought for plant population dynamics in tropical forests.





Similar content being viewed by others
References
APG (1998) An ordinal classification for the families of flowering plants. Ann Miss Bot Gard 85:531–553
Becker P (1992) Seasonality of rainfall and drought in Brunei Darussalam. Brunei Mus J 7:99–109
Becker P, Wong M (1993) Drought-induced mortality in tropical heath forest. J Trop Sci 5:416–417
Becker P, Rabenold PE, Idol JR, Smith AP (1988) Water potential gradients for gaps and slopes in a Panamanian tropical moist forest's dry season. J Trop Ecol 4:173-184
Bongers F, Poorter L, Van Rompaey RSAR, Parren MPE (1999) Distribution of twelve moist forest canopy tree species in Liberia and Cote d'Ivoire: response curves to a climatic gradient. J Veg Sci 10:371–382
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Brown ND, Whitmore TC (1992) Do dipterocarp seedlings really partition tropical rain forest gaps? Phil Trans R Soc Lond B 335:369–378
Burslem DFRP, Grubb PJ, Turner IM (1996) Responses to simulated drought and elevated nutrient supply among shade-tolerant tree seedlings of lowland tropical forest in Singapore. Biotropica 28:636–646
Caldwell MM, Dawson TE, Richards JH (1998) Hydraulic lift: consequences of water efflux from the roots of plants. Oecologia 113:151–161
Cao K-F (2000) Water relations and gas exchange of tropical saplings during a prolonged drought in a Bornean heath forest, with reference to root architecture. J Trop Ecol 16:101–116
Chandrashekar TR, Nazeer MA, Marattukalam JG, Prakash P, Annamalainathan K, Thomas J (1998) An analysis of growth and drought tolerance in rubber during the immature phase in a dry subhumid climate. Exp Agric 34:287–300
Condit R (1998) Ecological implications of changes in drought patterns: shift in forest composition in Panama. Clim Change 39:413–427
Condit R, Hubbell SP, Foster RB (1995) Mortality rates of 205 neotropical tree and shrub species and the impact of severe drought. Ecol Monogr 65:419–439
Croat TB (1978) The flora of Barro Colorado Island. Stanford University Press, Stanford
Currie DJ, Paquin V (1987) Largescale biogeographical patterns of species richness of trees. Nature 329:326–327
Engelbrecht BMJ, Herz H (2001) Evaluation of different methods to estimate understory light conditions in tropical forest. J Trop Ecol 17:207–224
Engelbrecht BMJ, Wright SJ, DeSteven D (2002) Effects of El Niño drought on survival and water relations of seedlings of three neotropical rainforest species in Panama. J Trop Ecol 18:569–579
Espigares T, Peco B (1995) Mediterranean annual pasture dynamics: impact of autumn drought. J Ecol 83:135–142
Evans CE, Etherington JR (1991) The effect of soil water potential on seedling growth of some British plants. New Phytol 118:571–579
Fernandez RJ, Reynolds JF (2000) Potential growth and drought tolerance of eight desert grasses: lack of a trade-off? Oecologia 123:90–98
Fisher BL, Howe HF, Wright SJ (1991) Survival and growth of Virola surinamensis yearlings: Water augmentation in gap and understory. Oecologia 86:292 –297
Garwood NC (1982) Seasonal rhythm of seed germination in a semideciduous tropical forest. In: Leigh EG Jr, Rand AS, Windsor DM (eds) The ecology of a tropical forest: seasonal rhythms and long-term changes. Smithsonian Institution Press, Washington, D.C., pp 173–199
Gentry AH (1988) Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann Miss Bot Gard 75:1–34
Gilbert GS, Harms KE, Hamill DN, Hubbell SP (2001) Effects of seedling size, El Niño drought, seedling density and distance to nearest conspecific adult on 6-year survival of Ocotea whitei seedlings in Panama. Oecologia 127:509–516
Gitay H, Noble IR (1997) What are functional types and how should we seek them? In: Smith TM, Shugart HH, Woodward FI (eds) Plant functional types. Their relevance to ecosystem properties and global change. (International Geosphere-Biosphere Programme book series) Cambridge University Press, Cambridge, pp 3–19
Givnish TJ (1999) On the causes of gradients in tropical tree diversity. J Ecol 87:193–210
Harms KE, Condit R, Hubbell SP, Foster RB (2001) Habitat associations of trees and shrubs in a 50-ha neotropical forest plot. J Ecol 89:947–959
Howe HF (1990) Survival and growth of juvenile Virola surinamensis in Panama: effects of herbivory and canopy closure. J Trop Ecol 6:259–280
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press. Princeton, N.J.
Hulme M, Viner D (1998) A climate change scenario for the tropics. Clim Change 39:145–176
Ito S, Nishiyama Y, Kustiawan W (2000) Responses of Dipterocarp seedlings to drought stress. In: Guhardja E, Fatawi M, Sutisna M, Mori T, Ohta S (eds.) Rainforest ecosystems of East Kalimantan. El Niño, drought, fire and human impacts. Springer, Berlin Heidelberg New York, pp 143–150
Jarvis PG, Jarvis MS (1963) The water relations of tree seedlings. 1. Growth and water use in relation to soil water potential. Physiol Plant 16:215–235
Kozlowsky TT, Pallardy SG (1997) Growth control of woody plants. Academic Press, San Diego, Calif.
Larcher W (1980) Physiological plant ecology, 2nd edn. Springer, Berlin Heidelberg New York
Lewis SL, Tanner EVJ (2000) Effects of aboveground and belowground competition on growth and survival of rain forest tree seedlings. Ecology 81:2525–2538
Lieth H (1975) Primary production of the major vegetation units of the world. In: Lieth H, Whittaker RH (eds) Primary productivity of the biosphere. Springer, Berlin Heidelberg New York, pp 203–231
Mulkey SS, Wright SJ (1996) Influence of seasonal drought on the carbon balance of tropical forest plants. In: Smith AP, Mulkey SS, Chazdon RL (eds) Tropical forest plant ecophysiology. Chapman and Hall, New York, pp 187–216
Nakagawa M, Tanaka K, Nakashizuka T, Ohkubo T, Kato T, Maeda T, Sato K, Miguchi H, Nagamasu H, Ogino K, Teo S, Hamid AA, Seng LH (2000) Impact of severe drought associated with the 1997–1998 El Niño in a tropical forest in Sarawak. J Trop Ecol 16:355–367
O'Brien EM (1993) Climatic gradients in woody plant species richness: towards an explanation based on an analysis of southern Africa's woody flora. J Biogeogr 20:181–198
Perneger TV (1998) What's wrong with Bonferroni adjustments? Br Med J 316:1236–1238
Poorter L, Hayashida-Oliver Y (2000) Effects of seasonal drought on gap and understory seedlings in a Bolivian moist forest. J Trop Ecol 16:481–498
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Richards PW (ed) (1998) The tropical rain forest: an ecological study, 2nd edn. Cambridge University Press, Cambridge
Schoeneweiss D (1986) Water stress disposition to disease—an overview. Water, fungi and plants. In: Ayres P (ed) Cambridge University Press, New York
Schupp EW (1988) Factors affecting post-dispersal seed survival in a tropical forest. Oecologia 76:525–530
Sollins P (1998) Factors influencing species composition in tropical lowland rainforest: Does soil matter? Ecology 79:23–30
Swaine MD (1996) Rainfall and soil fertility as factors limiting forest species distributions. J Ecol 84:419–428
Swaine MD, Whitmore TC (1988) On the definition of ecological species groups in tropical rain forests. Vegetatio 75:81–86
Timmermann A, Oberhuber J, Bacher A, Esch M, Latif M, Roeckner E (1999) Increased El Niño frequency in a climate model forced by future greenhouse warming. Nature 398:694–697
Tobin MF, Lopez OR, Kursar TA (1999) Responses of tropical understory plants to a severe drought: tolerance and avoidance of water stress. Biotropica 31:570–578
Toma T, Marjenah, Hastaniah (2000) Climate in Bukit Soeharto, East Kalimantan. In: Guhardja E, Fatawi M, Sutisna M, Mori T, Ohta S (eds) Rainforest ecosystems of East Kalimantan. El Niño, drought, fire and human impacts. Springer, Berlin Heidelberg New York, pp 107–117
Turner IM (1990) The seedling survivorship and growth of three Shorea species in a Malaysian tropical rain forest. J Trop Ecol 6:469–478
Tyree MT, Vargas G, Engelbrecht BMJ, Kursar TA (2002) Drought until death do us part: a case study of the desiccation-tolerance of a tropical moist forest seedling-tree, Licania platypus (Hemsl.) Fritsch. J Exp Bot 53:2239–2247
Unwin GL, Kriedemann PE (1990) Drought tolerance and rainforest tree growth on a North Queensland rainfall gradient. For Ecol Manage 30:113–123
VAST nomenclatural database (2002) Missouri Botanical Garden, St. Louis, Miss. http://mbot.org/W3T/search/vast.html 20
Veenendaal EM, Swaine MD (1998) Limits to tree species distribution in lowland tropical rainforests. In: Newbery DM, Prins HHT, Brown N (eds) Dynamics of tropical forest communities. Thirty-seventh Symposium of the British Ecological Society. Blackwell, Oxford, pp 163–191
Veenendaal EM, Swaine MD, Agyeman VK, Blay D, Abebrese IK, Mullins CE (1995) Differences in plant and soil water relations in and around a forest gap in West Africa during the dry season may influence seedling establishment and survival. J Ecol 83:83–90
Walsh RPD (1998) Climate. In: Richards PW (ed) The tropical rain forest: an ecological study, 2nd edn. Cambridge University Press, Cambridge, pp 159–202
Walsh RPD, Newbery DM (1999) The ecoclimatology of Danum, Sabah, in the context of the world's rainforest regions, with particular reference to dry periods and their impact. Phil Trans R Soc Lond B 354:1391–1405
Webb CO, Peart DR (2000) Habitat associations of trees and seedlings in a Bornean rain forest. J Ecol 88:464–478
Whitmore TC (1984) Tropical rainforests of the Far East, 2nd edn. Oxford University Press, Oxford
Windsor DM (1990) Climate and moisture availability in a tropical forest. Long-term records from Barro Colorado Island, Panama. Smithsonian Institution Press, Washington, D.C.
Wolda H (1988) Insect seasonality: why? Annu Rev Ecol Syst 19:1–18
Wright SJ (1996) Phenological responses to seasonality in tropical forest plants. In: Smith AP, Mulkey SS, Chazdon RL (eds) Tropical forest plant ecophysiology. Chapman and Hall, New York, pp 440–460
Wright SJ (2002) Plant diversity in tropical forests: a review of mechanisms of species coexistence. Oecologia 130:1–14
Acknowledgements
Maria del Carmen Ruiz, David Galvez and Didimo Moran provided essential assistance in all stages of the experiment. Additional assistance was provided by Robert Wolf, Beatriz Baker, Kelly Anderson, Eli Robbins, Ana Matilde Ruiz, Teresa Ruiz and Sebastian Brulez. We thank Mel Tyree for his suggestions throughout the study and for comments on the manuscript. Osvaldo de Leon, Andres Hernandez, Rolando Perez, Salomon Aguilar, Rafael Aizprua, Nayda Flores and Blanca Arauz provided expertise in seed and plant identification. This project was funded by the Andrew W. Mellon Foundation, and the University of Utah.
Author information
Authors and Affiliations
Corresponding author
Appendix 1.
Appendix 1.
Sample sizes at the beginning of the experiment for each treatment, number of survivors, and relative leaf-area changes (DLA) for all individuals (alive and dead)
The data are medians (minimum; maximum in parentheses). For abbreviations, see Table 1.
Species code | N | Alive | Leaf-area change | Mann-Whitney | |||
|---|---|---|---|---|---|---|---|
Wet | Dry | Wet | Dry | Wet | Dry | ||
AND | 30 | 29 | 18 | 12 | 0.000 | 0.000 | n.s. |
(0.000; 1.572) | (0.000; 1.235) | ||||||
BEI | 30 | 30 | 16 | 0 | 0.964 | 0.000 | - |
(0.000; 1.393) | (0.000; 0.000) | ||||||
CAL | 30 | 30 | 30 | 6 | 1.000 | 0.000 | *** |
(0.000; 1.533) | (0.000; 1.180) | ||||||
COR | 28 | 26 | 27 | 19 | 0.965 | 0.347 | *** |
(0.000; 2.315) | (0.000; 0.925) | ||||||
CUP | 30 | 31 | 29 | 26 | 1.079 | 0.957 | ** |
(0.000; 1.951) | (0.000; 1.377) | ||||||
DIP | 30 | 30 | 22 | 22 | 0.764 | 0.000 | ** |
(0.000; 4.789) | (0.000; 0.967) | ||||||
GAR | 30 | 30 | 30 | 29 | 1.000 | 1.000 | ** |
(0.949; 2.120) | (0.000; 1.000) | ||||||
HOR | 30 | 29 | 29 | 27 | 1.076 | 0.861 | *** |
(0.000; 1.803) | (0.000; 1.036) | ||||||
HYB | 30 | 31 | 30 | 26 | 1.228 | 0.368 | *** |
(0.107; 2.480) | (0.000; 1.306) | ||||||
HYM | 30 | 30 | 23 | 18 | 0.801 | 0.246 | * |
(0.000; 1.615) | (0.000; 1.155) | ||||||
ING | 30 | 30 | 25 | 23 | 1.039 | 0.235 | ** |
(0.000; 2.082) | (0.000; 1.190) | ||||||
LAC | 30 | 30 | 30 | 26 | 1.292 | 1.000 | ** |
(0.000; 1.821) | (0.000; 1.392) | ||||||
LAI | 30 | 30 | 30 | 16 | 1.412 | 0.805 | *** |
(0.000; 5.201) | (0.000; 1.222) | ||||||
LIC | 30 | 30 | 27 | 18 | 1.000 | 0.697 | ** |
(0.000; 1.410) | (0.000; 1.116) | ||||||
OUR | 30 | 30 | 30 | 29 | 1.372 | 1.013 | *** |
(0.293; 2.149) | (0.000; 1.310) | ||||||
PIC | 30 | 30 | 28 | 20 | 1.315 | 0.985 | ** |
(0.000; 2.300) | (0.000; 1.512) | ||||||
POU | 30 | 30 | 26 | 17 | 1.012 | 0.415 | ** |
(0.000; 2.533) | (0.000; 1.468) | ||||||
PSE | 30 | 30 | 27 | 24 | 0.471 | 0.000 | *** |
(0.000; 3.624) | (0.000; 0.079) | ||||||
PTE | 26 | 24 | 26 | 17 | 1.000 | 0.790 | ** |
(0.745; 3.056) | (0.000; 1.075) | ||||||
PTRI | 30 | 30 | 28 | 5 | 1.000 | 0.000 | *** |
(0.000; 1.528) | (0.000; 1.031) | ||||||
SOR | 30 | 30 | 30 | 16 | 1.000 | 0.081 | *** |
(0.553; 2.276) | (0.000; 1.296) | ||||||
SWA | 30 | 30 | 30 | 26 | 1.000 | 0.992 | n.s. |
(0.000; 1.634) | (0.000; 1.706) | ||||||
TAB | 30 | 30 | 27 | 12 | 0.651 | 0.000 | *** |
(0.000; 2.929) | (0.000; 0.526) | ||||||
THE | 30 | 29 | 27 | 20 | 1.163 | 0.081 | *** |
(0.000; 1.744) | (0.000; 1.682) | ||||||
TRC | 30 | 29 | 30 | 22 | 1.036 | 1.000 | *** |
(0.949; 2.081) | (0.000; 1.194) | ||||||
VIR | 30 | 30 | 29 | 6 | 1.000 | 0.000 | *** |
(0.000; 2.003) | (0.000; 1.000) | ||||||
XYL | 24 | 25 | 24 | 9 | 1.476 | 0.000 | *** |
(0.988; 2.729) | (0.000; 1.178) | ||||||
XYO | 27 | 25 | 27 | 20 | 1.000 | 0.705 | ** |
(0.182; 1.920) | (0.000; 1.000) | ||||||
Rights and permissions
About this article
Cite this article
Engelbrecht, B.M.J., Kursar, T.A. Comparative drought-resistance of seedlings of 28 species of co-occurring tropical woody plants. Oecologia 136, 383–393 (2003). https://doi.org/10.1007/s00442-003-1290-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1290-8